|
|
| Scientific Research | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
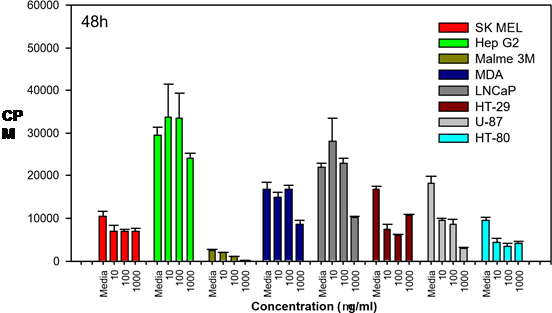
In 2007, KGK Synergize Inc., an independent
laboratory in Canada, examined the effects of POLY-MVA on
8
cancer cell lines.
These lines included: 1) Skin melanoma, human (SKMel-5) 2) Liver, hepatocellular carcinoma, human (Hep G2) 3) Lung, malignant melanoma, human (Malme-3M) 4) Mammary gland, ductal carcinoma, human (MDA-MB 435) 5) Prostate, left supraclavicular lymph node carcinoma, human (LNCaP) 6) Colon, colorectal adenocarcinoma, human (HT-29) 7) Human brain, glioblastoma; astrocytoma (U87) 8) Glioblastoma (HT-80) POLY-MVA was administered at 3 different dosages and the number of cells was examined after 24, 48 and 72 hours following initial application. POLY-MVA was effective, to varying degrees, on the entire group of cell lines tested (melanoma, liver, lung, breast, prostate, colon, astrocytoma and glioblastoma). . The varying effectiveness appears to be a consequence of the particular cell lines used and their associated degree of anaplasia.
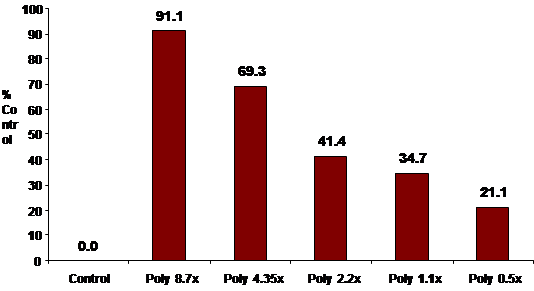
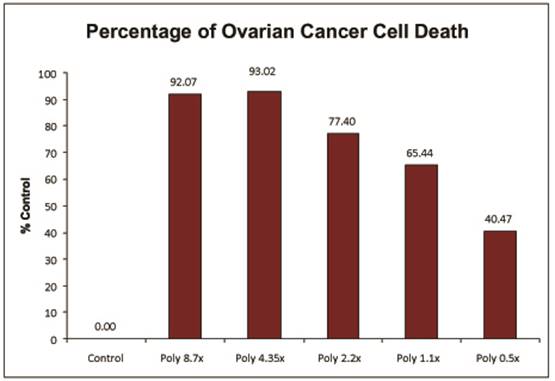
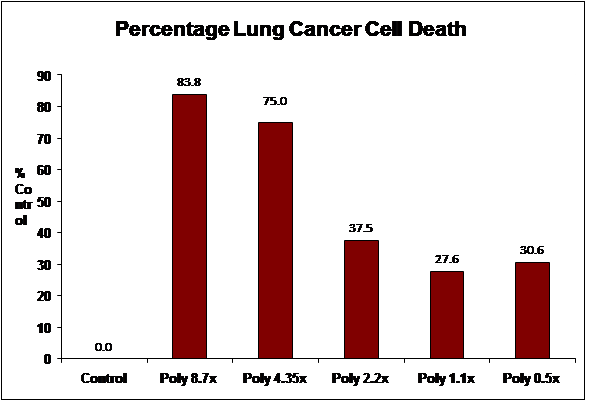
The following cell lines were selected from the NCI repository: MCF-7 (breast adenocarcinoma), and A549 (lung non-small cell adenocarcinoma).
The data below represents the completion of the Breast Cancer
(MCF-7), Ovarian Cancer (OVCAR-5) and Lung Carcinoma (A549) assay.
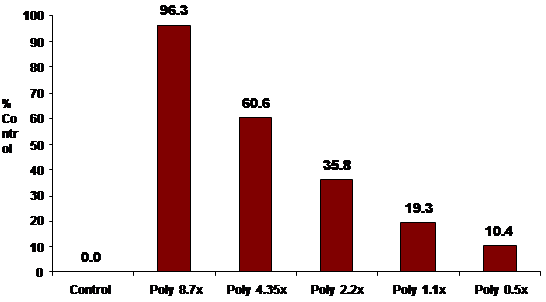
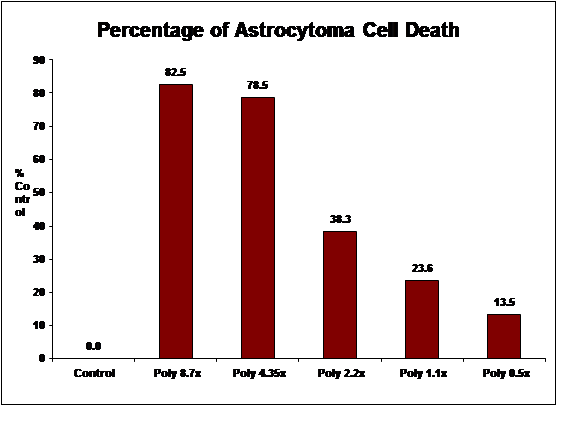
We have also completed assays using stage IV glioblastoma multiforme
(H-80) and astrocytoma (H-4) brain tumor lines. As noted below, all of the studies demonstrated significant cell death. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||